Submission to VIJ 2024-11-19
Copyright (c) 2024 Binitkumar M Vaghani

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
The medical device industry, highly regulated and sensitive to quality standards, relies on rigorous supplier audits to ensure compliance and mitigate risks. However, traditional supplier audits are often resource-intensive, inconsistent in quality, and lack a clear focus on the most critical risk factors. This paper explores how Artificial Intelligence (AI) can revolutionize the supplier audit process by enabling a risk-based approach that enhances accuracy, efficiency, and regulatory compliance.
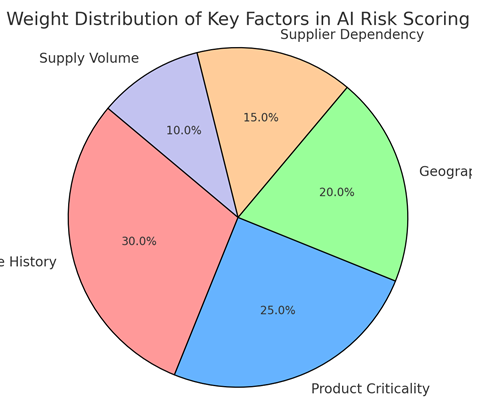
AI technology, with its advanced data processing, predictive analytics, and machine learning capabilities, can analyze vast amounts of supplier data in real-time, generating risk scores that prioritize high-risk suppliers for more frequent and thorough audits. This shift allows medical device companies to better allocate resources, focusing on high-impact areas and enhancing overall supply chain security. By considering factors such as supplier compliance history, product criticality, regional compliance laws, and performance trends, AI provides a dynamic and data-driven assessment model that minimizes the reliance on subjective audit practices.
The paper introduces an AI-powered risk-based audit framework specifically tailored for medical device companies. This framework utilizes a multi-faceted AI-driven risk scorecard, categorizing suppliers by risk levels (high, medium, low) and enabling targeted audit strategies. It also demonstrates how AI can process a wide array of audit-related data inputs—such as supplier compliance, regional geopolitical risks, supply volume, and product criticality—to generate a holistic and accurate risk assessment.
Through case studies and industry examples, this research underscores the advantages of integrating AI into supplier audit processes, highlighting cost savings, improved compliance outcomes, and reduced risk of quality issues or product recalls. Furthermore, it examines future trends and ethical considerations of AI in supplier audits, advocating for industry-wide adoption of AI-powered tools to support more efficient and effective supplier management.
This paper concludes that adopting a risk-based, AI-driven audit approach in the medical device sector not only supports regulatory compliance but also builds resilience within the supply chain, creating a safer and more efficient landscape for medical device production.
References
- Fraser, A. G., Biasin, E., Bijnens, B., Bruining, N., Caiani, E. G., Cobbaert, K., ... & Rademakers, F. E. (2023). Artificial intelligence in medical device software and high-risk medical devices–a review of definitions, expert recommendations and regulatory initiatives. Expert Review of Medical Devices, 20(6), 467-491.
- Nikfal, N. (2024). A DEVELOPMENT PROCESS FRAMEWORK FOR ARTIFICIAL INTELLIGENCE/MACHINE LEARNING (AI/ML)-BASED CONNECTED HEALTH INFORMATICS (Doctoral dissertation, Purdue University Graduate School).
- Castka, P., Zhao, X., Bremer, P., Wood, L. C., & Mirosa, M. (2022). Supplier audits during COVID-19: a process perspective on their transformation and implications for the future. The International Journal of Logistics Management, 33(4), 1294-1314.
- Jabri, S. (2020). Artificial intelligence and healthcare: products and procedures. Regulating artificial intelligence, 307-335.
- Mökander, J. (2023). Ethics-based auditing of automated decision-making systems: considerations, challenges, na (Doctoral dissertation, University of Oxford).
- Beckers, R., Kwade, Z., & Zanca, F. (2021). The EU medical device regulation: Implications for artificial intelligence-based medical device software in medical physics. Physica Medica, 83, 1-8.
- Odaibo, S. G. (2021). Risk Management of AI/ML Software as a Medical Device (SaMD): On ISO 14971 and Related Standards and Guidances. arXiv preprint arXiv:2109.07905.
- Abd Rahman, N. H., Hasikin, K., Abd Razak, N. A., Al-Ani, A. K., Anni, D. J. S., & Mohandas, P. (2023). Medical device failure predictions through AI-Driven analysis of Multimodal Maintenance Records. IEEE Access, 11, 93160-93179.
- Mkwashi, A., & Brass, I. (2022). The Future of Medical Device Regulation and Standards: Dealing with Critical Challenges for Connected, Intelligent Medical Devices. PETRAS National Centre of Excellent in IoT Systems Cybersecurity: London (2022), DOI, 10.
- Wellnhofer, E. (2022). Real-world and regulatory perspectives of artificial intelligence in cardiovascular imaging. Frontiers in Cardiovascular Medicine, 9, 890809.
- Abedsoltan, H., & Abedsoltan, A. (2024). Future of process safety: Insights, approaches, and potential developments. Process Safety and Environmental Protection.
- Czekster, R. M., Grace, P., Marcon, C., Hessel, F., & Cazella, S. C. (2023). Challenges and opportunities for conducting dynamic risk assessments in medical IoT. Applied Sciences, 13(13), 7406.
- Mokander, J., & Floridi, L. (2024). Operationalising AI governance through ethics-based auditing: an industry case study. arXiv preprint arXiv:2407.06232.
- Wijesooriya, C., & Basnayake, R. (2024). Digital Transformation in Redefining the Role of the Finance and Audit Professional of the Future. In Digital Transformation in Accounting and Auditing: Navigating Technological Advances for the Future (pp. 61-104). Cham: Springer International Publishing.
- Gupta, R. K., Cherukuri, H., Shukla, S., Rajan, A. T., & Aravind, S. (2024). Deploying Containerized Microservices in on-Premise Kubernetes Environments: Challenges and Best Practices. International Journal of Multidisciplinary Innovation and Research Methodology, ISSN: 2960-2068, 3(2), 74-90.
- Mammadzada, A. Evolving Environmental Immigration Policies Through Technological Solutions: A Focused Analysis of Japan and Canada in the Context of COVID-19.
- Bhat, P., Shukla, T., Naik, N., Korir, D., Princy, R., Samrot, A. V., ... & Salmataj, S. A. (2023). Deep Neural Network as a Tool to Classify and Identify the 316L and AZ31BMg Metal Surface Morphology: An Empirical Study. Engineered Science, 26, 1064.
- Nie, P., Parovic, M., Zang, Z., Khurshid, S., Milicevic, A., & Gligoric, M. (2020). Unifying execution of imperative generators and declarative specifications. Proceedings of the ACM on Programming Languages, 4(OOPSLA), 1-26.
- SHUKLA, T. (2024). Beyond Diagnosis: AI’s Role in Preventive Healthcare and Early Detection.
- Zang, Z., Wiatrek, N., Gligoric, M., & Shi, A. (2022, October). Compiler testing using template java programs. In Proceedings of the 37th IEEE/ACM International Conference on Automated Software Engineering (pp. 1-13).
- Das, A., Shukla, T., Tomita, N., Richards, R., Vidis, L., Ren, B., & Hassanpour, S. (2024). Deep Learning for Classification of Inflammatory Bowel Disease Activity in Whole Slide Images of Colonic Histopathology. arXiv preprint arXiv:2410.19690.
- Ghelani, H. (2024). AI-Driven Quality Control in PCB Manufacturing: Enhancing Production Efficiency and Precision. Valley International Journal Digital Library, 1549-1564.
- Chanane, F. (2024). Exploring Optimization Synergies: Neural Networks and Differential Evolution for Rock Shear Velocity Prediction Enhancement. International Journal of Earth Sciences Knowledge and Applications, 6(1), 21-28.
- Buhse, B., Wei, T., Zang, Z., Milicevic, A., & Gligoric, M. (2019, May). Vedebug: regression debugging tool for java. In 2019 IEEE/ACM 41st International Conference on Software Engineering: Companion Proceedings (ICSE-Companion) (pp. 15-18). IEEE.
- Ghelani, H. (2024). Advanced AI Technologies for Defect Prevention and Yield Optimization in PCB Manufacturing. Valley International Journal Digital Library, 26534-26550.
- Miloud, M. O. B., & Liu, J. (2023, April). An Application Service for Supporting Security Management In Software-Defined Networks. In 2023 7th International Conference on Cryptography, Security and Privacy (CSP) (pp. 129-133). IEEE.
- Ghelani, H. (2021). Advances in lean manufacturing: improving quality and efficiency in modern production systems. Valley International Journal Digital Library, 611-625.
- Lakhani, R. (2023). Cybersecurity Threats in Internet of Things (IoT) Networks: Vulnerabilities and Defense Mechanisms. Valley International Journal Digital Library, 25965-25980.
- Lakhani, R., & Sachan, R. C. (2024). Securing Wireless Networks Against Emerging Threats: An Overview of Protocols and Solutions.
- Lakhani, R. Zero Trust Security Models: Redefining Network Security in Cloud Computing Environments.